Wholesale Cfda Approved Skin Icterus Tester
Hunan Runmei Gene Technology Co., Ltd.- Type:No-Needle Mesotherapy Device
- Theory:Laser
- Application:Salon, Home
- Portable:Portable
- Transport Package:Carton
- Trademark:Runmei
Base Info
- Model NO.:BY-D-I
- Origin:China
Description
Basic Info.
Model NO. BY-D-I Origin ChinaProduct Description
Product Parameters Product parameter information, packaging list.Specification and model type BY-D-I
Measurement is reflected by specific wavelength light, blue light wave (460nm), green
Optical wave (550nm) comparison
Display the method LCD display
Display value error 00~15 ± 1mg/dL, 16~25 ± 1.5mg/dL Precision RSD <2%
Xenon flash light source
Power # 71.2V Nickel 22 Charging Battery 4 measurements for each new battery replacement, measuring approximately 157g(with battery pack) size (mm) 175 (long) × 68 (width) × 26 (thick)
The check disc displays 00.0mg/dl or 00.1mg/dl on the white screen and on yellow
The screen displays the 20.0 ± 0.5mg/dl
Packaging list Metal packing box manual Host certificate of qualification verification plate Package repair card charger wipe cloth qualification certificate
* Under the testing environment and instruments developed by Boko, using differen
Company Profile:
Hunan Runmei Gene Technology Co., Ltd is a high-tech enterprise dedicated to the
development of the gene detection products and the construction of big data service
platforms led by a team of doctors at home and abroad. Our company's strategic goal is
to base on China and radiate the world, and to solve the pain points and difficulties of the
industry and create value for human beings as our corporate purpose. At present, our
company has completed the construction of product systems for pathogen biology fluoresence
quantitative PCR detection kits, pathogen biology ELISA detection kits and pathogen biology
immune colloidal gold detection kits.



Certification:




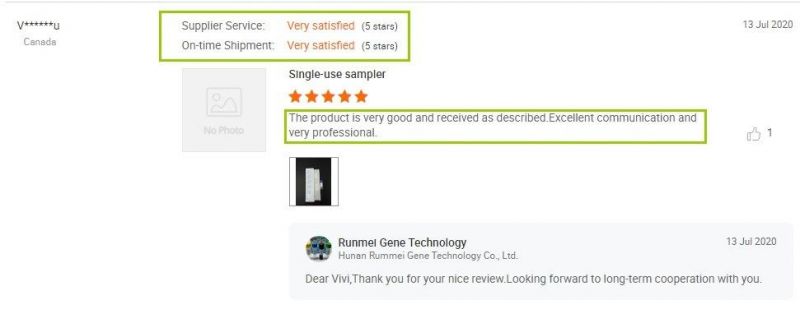
Thank you for our Canadian customer's 5 star Review: